Roots Analysis
Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market Research Report 2019-2030
Given the invasive and cost-intensive nature of tissue biopsies, there is a significant unmet need for safer and more patient-friendly cancer diagnostics that are capable of offering highly accurate, and actionable insights related to the disease.
Get Detailed Analysis of 350+ page report, which features 150+ figures and 200+ tables,
The financial opportunity within the liquid biopsy and other non-invasive cancer diagnostics market has been analyzed across the following segments:
Type of Tumor Marker
- ctDNA
- cfDNA
- CTCs
- Exosomes
- Others
Application
- Diagnosis / Early Diagnosis
- Patient Monitoring
- Recurrence Monitoring
Target Cancer Indication
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Bladder Cancer
- Melanoma
- Gastric Cancer
- Pancreatic Cancer
- Ovarian Cancer
- Others
End Users
- Hospitals
- Research Institutes
- Others
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Request for Sample: https://www.rootsanalysis.com/reports/view_document/liquid-biopsy-and-nicd-market/279.html
The Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market (3rd Edition), 2019-2030: Focus on Circulating Tumor Markers such as CTCs, ctDNA, cfDNA, Exosomes and Other Biomarkers report features the following companies, which we identified to be key players in this domain:
Key Players:
- Amoy Diagnostics
- DiaCarta
- HaploX Biotechnology
- NeoGenomics
- QIAGEN
- Swift Biosciences
- Sysmex Inostics
- Thermo Fisher Scientific
Table of Contents
- Preface
- Executive Summary
- Introduction
- Non-Invasive Cancer Screening and Diagnosis
- Market Landscape
- Company Profiles
- Partnerships and Collaborations
- Funding and Investment Analysis
- Liquid Biopsy: Initiatives of Big Pharma Players
- Key Acquisition Targets
- Other Non-Invasive Cancer Diagnostics
- Market Sizing and Opportunity Analysis
- Survey Insights
- Conclusion
- Executive Insights
- Appendix 1: Tabulated Data
- Appendix 2: List of Companies and Organizations
To purchase a copy, please visit https://www.rootsanalysis.com/reports/view_document/liquid-biopsy-and-nicd-market/279.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Cell and Advanced Therapies Supply Chain Management Market Size, Analysis, Opportunities and Trends 2019 –2030
Advanced therapy medicinal products, such as cell and gene therapies, have revolutionized healthcare practices. The introduction of such treatment options has led to a paradigm shift in drug development, production and consumption.
Get Detailed Analysis at: https://www.rootsanalysis.com/reports/view_document/cell-therapies-supply-chain/260.html
Key Inclusions
- A detailed assessment of the current market landscape, featuring a comprehensive list of over 160 technological platforms that are being used to manage the cell and advanced therapies supply chain, along with information on the different types of software systems (COP, EMS, IMS, LIMS, LMS, PMS, QMS, TTS, and others), their key specifications and benefits (chain of identity and custody, compatibility and integration, data management and analytics, regulatory compliance, reliability and security, scalability, software-as-a-service, traceability, user-friendliness, workflow management, and others), affiliated modes of deployment (cloud and on-premises), scale of management (small enterprise, mid-size enterprise and large enterprise), end users (biobanks, cell therapy labs, hospitals, research institutes, commercial organizations, and others), applications (ordering and scheduling, sample collection, manufacturing, logistics, and patient verification and treatment follow-up), regulatory certifications / accreditations (21 CFR Part 11, CLIA, FACT-JACIE, GAMP 5, GDPR, HIPAA, and others), and key support services offered (customization, installation / implementation, maintenance, training / technical support, upgradation, validation and testing, and others).
- An insightful company competitiveness analysis, taking into consideration the supplier power (based on their employee base and years of experience in the industry) and portfolio-related parameters, such as number of software solutions offered, affiliated modes of deployment, scale of management, end users, applications, regulatory certifications / accreditations, support services offered, and key platform specifications and benefits.
- Comprehensive profiles of industry players that are currently offering software solutions for supply chain management, featuring an overview of the company, its financial information (if available), and a detailed description of its software system(s). Each profile also includes a list of recent developments, highlighting the key achievements, partnership activity, and the likely strategies that may be adopted by these players to fuel growth, in the foreseen future.
- A detailed review of the cell and advanced therapies supply chain, offering insights on the processes associated with various stages, such as donor eligibility assessment, sample collection, manufacturing, logistics, and patient verification and treatment follow-up, along with information on cost requirements and existing opportunities for improvement in the supply chain management practices.
- A qualitative assessment of the current and long-term needs of different stakeholders (patients, healthcare providers, collection centers, manufacturers, logistics service providers and regulators / payers) involved in the cell and advanced therapies supply chain, featuring a summary of the diverse needs and areas of concern, along with our opinion (based on past and prevalent trends) on how the industry is preparing to address such issues.
- An analysis of the investments made at various stages of development, such as seed financing, venture capital financing, debt financing, grants, capital raised from IPOs and subsequent offerings received by companies that are engaged in this field.
- An analysis of the partnerships that have been established in the domain, in the period between 2014 and Q3 2019, covering software licensing agreements, mergers and acquisitions, product development agreements, product integration agreements, distribution agreements, asset purchase agreements, and other relevant deals.
- A detailed analysis of the platform utilization use cases where aforementioned software systems were leveraged by various stakeholders in the domain, in the period between 2014 and Q3 2019, highlighting the ways in which companies have implemented such systems to improve / optimize various supply chain-related processes of cell and advanced therapies.
- An in-depth analysis of the cost saving potential across various processes of the cell and advanced therapies supply chain that can be brought about by the implementation of bespoke and integrated technological solutions / software systems.
- A case study on COPs, featuring insights on their key functions and implementation strategies, while also considering their strategic position and connectivity with other adjacent systems within the cell and advanced therapies supply chain. In addition, it provides a brief discussion on the growing popularity of COPs on the social media platform, Twitter.
The USD 1.5 billion (by 2030) financial opportunity within the cell and advanced therapies supply chain management market has been analysed across the following segments:
https://www.rootsanalysis.com/reports/view_document/cell-therapies-supply-chain/260.html
Application area
- Sample collection and processing
- Manufacturing
- Logistics
- Patient identification and treatment follow-up
Type of software solution
- Cell orchestration platform
- Enterprise manufacturing system
- Inventory management system
- Laboratory information management system
- Logistics management system
- Patient management system
- Quality management system
Mode of Deployment
- Cloud-based solution
- On-premises solution
Scale of Operation
- Clinical
- Commercial
End user
- Biobank
- Cell therapy lab / commercial organization
- Hospital / medical center
- Research institute
Key geographical regions
- North America
- Europe
- Asia Pacific
- Rest of the world
For more info: https://www.rootsanalysis.com/reports/view_document/cell-therapies-supply-chain/260.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Target Protein Degradation Market is Expected to Reach 3.6 billion by 2030
The concept of targeted protein degradation presents revolutionary drug development opportunities and is anticipated to bring about a paradigm shift in modern healthcare. The first targeted protein degrader, called proteolysis targeting chimera (PROTAC), was developed about a decade ago.
Presently, a variety of other such chemical entities and molecular glues are under investigation. In fact, certain pipeline candidates are already in the mid to late-phase trials and are anticipated to soon enter the market.
The USD 3.6 billion (by 2030) financial opportunity within the Target Protein Degradation Market has been analyzed across the following segments:
Type of payment of licensing agreements
- Upfront payments
- Milestone payments
Type of protein degrader
- Degronimids
- PROTACs
- SARDs / SERDs
- Specific BET and DUB inhibitors
- Other protein degraders
Therapeutic area
- Neurodegenerative disorders
- Oncological disorders
- Other therapeutic areas
Route of administration
- Oral
- Intravenous
- Other routes
Key geographical region
- North America
- Europe
- Asia-Pacific
The Targeted Protein Degradation Market: Focus on Therapeutics and Technology Platforms (based on Degronimids, ENDTACs, Epichaperome Inhibitors, Hydrophobic Tags, IMiDs, LYTACs, Molecular Glues, PHOTACs, PROTACs, Protein Homeostatic Modulators, SARDs, SERDs, SNIPERs, and Specific BET and DUB Inhibitors), 2020-2030 report features the following companies, which we identified to be key players in this domain:
Get detailed Analysis: https://www.rootsanalysis.com/reports/view_document/protein-degradation-market/289.html
Key Players
- Arvinas
- Captor Therapeutics
- Celgene
- Genetech
- Kymera Therapeutics
- Mission Therapeutics
- Progenra
- Radius Health
- Sanofi Genzyme
- Zenopharm
Table of Contents
- Preface
- Executive Summary
- Introduction
- Current Market Landscape
- Company Profiles
- Clinical Trial Analysis
- KOL Analysis
- Publication Analysis
- Funding and Investment Analysis
- Partnerships and Collaborations
- Market Sizing and Opportunity Analysis
- Executive Insights
- Concluding Remarks
- Appendix 1: Tabulated Data
- Appendix 2: List of Companies and Organizations
To purchase a copy, please visit https://www.rootsanalysis.com/reports/view_document/protein-degradation-market/289.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Subcutaneous Biologic Drugs And Affiliated Technologies Market Research Report, Forecast to 2030 | Roots Analysis
Roots Analysis has done a detailed report on Subcutaneous Biologics, Technologies and Drug Delivery Systems (3rd Edition), 2020-2030 covering key aspects of the industry and identifying future growth opportunities.
Key Market Insights
- Eminent representatives from biopharmaceutical companies confirm the rising interest in the concept of subcutaneous drug delivery, highlighting some of the key drivers and upcoming trends in this domain
- More than 100 subcutaneous biologics have been approved and over 350 such drug candidates are being evaluated in the clinical stages of development, for the treatment of a wide variety of disease indications
- Antibodies and protein therapeutics represent the majority of subcutaneous biologics that are available / under investigation, designed for use against various therapeutic areas and having different dosing regimens
- Advances in drug delivery have led to the development of novel technology platforms, enabling the administration of highly viscous formulations, and supporting the development of subcutaneous dosage forms
- Several technology developers have out-licensed their proprietary platforms to pharmaceutical companies in order to enable them to develop subcutaneous formulations of their approved / pipeline products
- The increasing interest in this field is reflected in the yearly growth in partnership activity, including a number of licensing and product development deals related to subcutaneous formulations of various drug candidates
- There are several new and innovative drug delivery systems that facilitate subcutaneous administration; we identified over 300 such systems that are presently available / under development
- With several self-medication enabling devices, such as wearable injectors and autoinjectors, available in the market, developers are actively differentiating their offerings by incorporating advanced, patient-friendly features
- The market is anticipated to be worth over USD 180 billion in 2030; the projected opportunity is likely to be distributed across various types of molecules that are developed / being developed for different disease indications
- Pre-filled syringes continue to dominate the current market of subcutaneous drug delivery systems; technology developers are expected to continue relying on licensing agreements as their primary source of revenues.
For more information, please visit https://www.rootsanalysis.com/reports/view_document/subcutaneous-biologics-delivery/314.html
Table of Contents
- PREFACE
- Scope of the Report
- Research Methodology
- Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
- Chapter Overview
- Types of Therapeutic Molecules
- Biologically Derived Therapeutics
- Types of Products
- Routes of Administration and Formulations
- Subcutaneous Formulations
- Approaches in Subcutaneous Delivery of Biologics
- Reformulation
- Differing Potencies
- Novel Technologies
- Method of Subcutaneous Administration
- Advantages of Subcutaneous Administration
- Limitations of Subcutaneous Administration
- Approaches in Subcutaneous Delivery of Biologics
- Regulatory Considerations
- Medical Devices
- Drug Device Combination Products
- Future Perspectives
- SUBCUTANEOUS BIOLOGICS: CURRENT MARKET LANDSCAPE
- Chapter Overview
- Subcutaneous Administration of Biologics
- Subcutaneous Biologics: List of Approved Drugs
- Analysis by Approval Year
- Analysis by Type of Pharmacological Molecule
- Analysis by Target Therapeutic Area
- Analysis by Type of Formulation
- Analysis by Dosing Frequency
- Analysis by Dosage Form
- Key Players: Analysis by Number of Drugs Approved
- Subcutaneous Biologics: List of Clinical-Stage Drug Candidates
- Analysis by Phase of Development
- Analysis by Type of Pharmacological Molecule
- Analysis by Target Therapeutic Area
- Analysis by Dosing Frequency
- Key Players: Analysis by Number of Drug Candidates in Trials
- CASE STUDY: LEADING SUBCUTANEOUS BIOLOGICS
- Chapter Overview
- Subcutaneous Biologics: Leading Drugs by Annual Sales
- Case Studies
- HUMIRA® (AbbVie, Eisai)
- Drug Overview
- HUMIRA® (AbbVie, Eisai)
- 3.1.2. Development History
- 3.1.3. Target Indications and Dosage Forms
- 3.1.4. Historical Sales
- Enbrel® (Amgen, Pfizer, Takeda Pharmaceutical)
- Overview
- 3.2.2. Development History
- 3.2.3. Target Indications and Dosage Forms
- 3.2.4. Historical Sales
- RITUXAN® / MabThera® (Biogen, Roche, Chugai Pharmaceutical)
- Overview
- 3.3.2. Development History
- 3.3.3. Target Indications and Dosage Forms
- 3.3.4. Historical Sales
- 3.3.5. ENHANZE™ Technology (Halozyme Therapeutics)
- 3.3.6. Advantages of Subcutaneous RITUXAN® / MabThera® Over Intravenous RITUXAN® / MabThera®
- Herceptin® (Roche, Chugai Pharmaceutical)
- Overview
- 3.4.2. Development History
- 3.4.3. Target Indications and Dosage Forms
- 3.4.4. Historical Sales
- 3.4.5. ENHANZE™ Technology (Halozyme Therapeutics)
- 3.4.6. Advantages of Subcutaneous Herceptin® Over Intravenous Herceptin®
- 3.4.7. Herceptin® - Large Volume Wearable Injector Combination Product
- Neulasta® (Amgen, Kyowa Hakko Kirin)
- Overview
- Development History
- Target Indications and Dosage Forms
- Historical Sales
- SUBCUTANEOUS FORMULATION TECHNOLOGIES: CURRENT MARKET LANDSCAPE
- Chapter Overview
- Subcutaneous Formulation Technologies: List of Technology Developers
- Analysis by Year of Establishment
- Analysis by Company Size
- Analysis by Geographical Location
- Subcutaneous Formulation Technologies: List of Technologies
- Analysis by Type of Pharmacological Molecule
- Analysis by Route of Administration
- Analysis by Advantage(s) Offered
- SUBCUTANEOUS FORMULATION TECHNOLOGY DEVELOPERS: COMPANY COMPETITIVENESS ANALYSIS
- Chapter Overview
- Subcutaneous Formulation Technology Developers: Competitive Landscape
- Methodology
- Three-Dimensional Bubble Analysis based on Supplier Power, Pipeline Strength and Primary Advantage(s)
- Subcutaneous Formulation Technology Developers: Benchmark Analysis
- Methodology
- North America
- Europe
- SUBCUTANEOUS FORMULATION TECHNOLOGY DEVELOPERS: COMPANY PROFILES
- Chapter Overview
- Adocia
- Company Overview
- Technology Overview
- BioChaperone® Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Ajinomoto Althea
- Company Overview
- Technology Overview
- Crystalomics® Formulation Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Arecor
- Company Overview
- Technology Overview
- Arestat™ Technology
- Product Portfolio
- Recent Developments and Future Outlook
- Alteogen
- Company Overview
- Technology Overview
- Hybrozyme Technology
- Product Portfolio
- Recent Developments and Future Outlook
- Ascendis Pharma
- Company Overview
- Technology Overview
- TransCon Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Avadel Pharmaceuticals
- Company Overview
- Technology Overview
- Medusa™ Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Camurus
- Company Overview
- Technology Overview
- FluidCrystal® Injection Depot Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Creative BioMart
- Company Overview
- Technology Overview
- High Concentration Formulation Technology
- Product Portfolio
- Recent Developments and Future Outlook
- Creative Biolabs
- Company Overview
- Technology Overview
- Long-Acting Injectable Technology
- Product Portfolio
- Recent Developments and Future Outlook
- DURECT
- Company Overview
- Technology Overview
- SABER® Platform
- CLOUD™ PLATFORM
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Eagle Pharmaceuticals
- Company Overview
- Technology Overview
- Unnamed Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- Halozyme Therapeutics
- Company Overview
- Technology Overview
- ENHANZE® Technology
- Product Portfolio
- Financial Performance
- Recent Developments and Future Outlook
- MedinCell
- Company Overview
- Technology Overview
- BEPO® Technology
- Product Portfolio
- Recent Developments and Future Outlook
- Xeris Pharmaceuticals
- Company Overview
- Technology Overview
- XeriJect™ Technology
- XeriSol™ Technology
- Product Portfolio
- Recent Developments and Future Outlook
- Serina Therapeutics
- Company Overview
- Technology Overview
- POZ™ Drug Delivery Technology
- Product Portfolio
- Recent Developments and Future Outlook
- PARTNERSHIPS AND COLLABORATIONS
- Chapter Overview
- Partnership Models
- Subcutaneous Formulation Technologies: Partnerships and Collaborations
- Analysis by Year of Partnership
- Analysis by Type of Partnership
- Most Active Players: Analysis by Number of Partnerships
- Regional Analysis
- Intercontinental and Intracontinental Agreements
- SUBCUTANEOUS DRUG DELIVERY SYSTEMS: CURRENT MARKET LANDSCAPE
- Chapter Overview
- Different Types of Subcutaneous Drug Delivery Systems
- Subcutaneous Drug Delivery Systems: Overall Market Landscape
- Large Volume Wearable Injectors
- Overview
- Current Market Landscape of Devices for Non-insulin Biologics
- Analysis by Stage of Development
- Analysis by Type of Device
- Analysis by Type of Dose Administered
- Analysis by Volume / Storage Capacity
- Analysis by Usability
- Analysis by Mode of Injection
- Analysis by Mechanism of Action
- Most Active Players: Analysis by Number of Devices
- Product Competitiveness Analysis
- Current Market Landscape of Devices for Insulin
- Analysis by Stage of Development
- Analysis by Type of Device
- Analysis by Volume / Storage Capacity
- Analysis by Usability
- Analysis by Availability of Integrated CGM / BGM System
- Most Active Players: Analysis by Number of Devices
- Large Volume Wearable Injectors
- SWOT ANALYSIS
- Chapter Overview
- Comparison of SWOT Factors
- Strengths
- Weaknesses
- Opportunities
- Threats
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
- Chapter Overview
- Subcutaneous Biologics Market
- Forecast Methodology and Key Assumptions
- Overall Subcutaneous Biologics Market, 2020-2030
- Subcutaneous Biologics Market, 2020-2030:Distribution by Phase of Development
- Subcutaneous Biologics Market, 2020-2030:Distribution by Type of Pharmacological Molecule
- Subcutaneous Biologics Market, 2020-2030:Distribution by Target Therapeutic Area
- Subcutaneous Biologics Market, 2020-2030:Distribution by Key Geographical Regions
- Subcutaneous Drug Delivery Systems Market
- Device Type 1: Large Volume Wearable Injectors
- Forecast Methodology and Key Assumptions
- Global Large Volume Wearable Injectors Market for Non-Insulin Drugs, 2020-2030
- Global Large Volume Wearable Injectors Market for Non-Insulin Drugs: Distribution by Type of Device, 2020-2030
- Global Large Volume Wearable Injectors Market for Non-Insulin Drugs: Distribution by Usability, 2020-2030
- Global Large Volume Wearable Injectors Market for Non-Insulin Drugs: Distribution by Target Therapeutic Area, 2020-2030
- Global Large Volume Wearable Injectors Market for Non-Insulin Drugs: Distribution by Key Geographical Regions, 2020-2030
- Global Large Volume Wearable Injectors Market for Insulin , 2020-2030
- Global Large Volume Wearable Injectors Market for Insulin: Distribution by Type of Device, 2020-2030
- Global Large Volume Wearable Injectors Market for Insulin: Distribution by Usability, 2020-2030
- Global Large Volume Wearable Injectors Market for Insulin: Distribution by Key Geographical Regions, 2020-2030
- Device Type 2: Autoinjectors
- Forecast Methodology and Key Assumptions
- Global Autoinjectors Market, 2020-2030
- Global Autoinjectors Market: Distribution by Usability, 2020-2030
- Global Autoinjectors Market: Distribution by Type of Pharmacological Molecule, 2020-2030
- Global Autoinjectors Market: Distribution by Key Geographical Regions, 2020-2030
- Device Type 3: Prefilled Syringes
- Forecast Methodology and Key Assumptions
- Global Prefilled Syringes Market, 2020-2030
- Global Prefilled Syringes Market: Distribution by Type of Syringe Barrel Material, 2020-2030
- Global Prefilled Syringes Market: Distribution by Type of Chamber System, 2020-2030
- Global Prefilled Syringes Market: Distribution by Type of Pharmacological Molecule, 2020-2030
- Global Prefilled Syringes Market: Distribution by Target Therapeutic Area, 2020-2030
- Global Prefilled Syringes Market: Distribution by Key Geographical Regions, 2020-2030
- Device Type 4: Needle-Free Injection Systems
- Forecast Methodology and Key Assumptions
- Global Needle-Free Injection Systems Market, 2020-2030
- Global Needle-Free Injection Systems Market: Distribution by Usability, 2020-2030
- Global Needle-Free Injection Systems Market: Distribution by Actuation Mechanism, 2020-2030
- Global Needle-Free Injection Systems Market: Distribution by Target Therapeutic Area, 2020-2030
- Global Needle-Free Injection Systems Market: Distribution by Key Geographical Regions, 2020-2030
- Device Type 5: Novel Drug Reconstitution Systems
- Forecast Methodology and Key Assumptions
- Global Novel Drug Reconstitution Systems Market, 2020-2030
- Subcutaneous Formulation Technologies Market
- Subcutaneous Formulation Technologies Market: Distribution by Upfront and Milestone Payments, 2020-2030
- Device Type 1: Large Volume Wearable Injectors
- CONCLUDING REMARKS
- EXECUTIVE INSIGHTS
- Chapter Overview
- Lindy Biosciences
- Company Snapshot
- Interview Transcript: Deborah Bitterfield, Chief Executive Officer and Founder
- Oval Medical Technologies
- Company Snapshot
- Interview Transcript: Matthew Young, Chief Technology Officer and Founder
- Xeris Pharmaceuticals
- Company Snapshot
- Interview Transcript: Steve Prestrelski, Chief Scientific Officer and Founder; Hong Qi, Vice President, Product Development; and Scott Coleman, Sr. Scientist Formulation)
- DALI Medical Devices
- Company Snapshot
- Interview Transcript: David Daily, Chief Executive Officer and Co-Founder
- Excelse Bio
- Company Snapshot
- Interview Transcript: Michael Reilly, Chief Executive Officer and Co-Founder
- i-novion
- Company Snapshot
- Interview Transcript: Poonam R Velagaleti, Co-Founder
- Enable Injections
- Company Snapshot
- Interview Transcript: Michael Hooven, Chief Executive Officer
- Immunovaccine Technologies
- Company Snapshot
- Interview Transcript: Frederic Ors, Chief Executive Officer
- Portal Instruments
- Company Snapshot
- Interview Transcript: Patrick Anquetil, Chief Executive Officer
- Elcam Medical
- Company Snapshot
- Interview Transcript: Menachem Zucker, Vice President and Chief Scientist
- West Pharmaceutical Services
- Company Snapshot
- Interview Transcript: Tiffany H Burke, Director, Global Communications and Graham Reynolds, Vice President and General Manager, Global Biologics
- MedinCell
- Company Snapshot
- Interview Transcript: David Heuzé, Communication Leader
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATION
To purchase a copy, please visit https://www.rootsanalysis.com/reports/view_document/subcutaneous-biologics-delivery/314.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Medical Device Label Contract Manufacturing Market is anticipated to grow at a CAGR 9.8% by 2030
Roots Analysis has done a detailed study on Medical Device Labels Technology Market, 2019-2030, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
Key Market Insights
- More than 80 companies presently claim to have the required expertise to design and manufacture different types of labels, using both conventional advanced printing methods, for use in medical devices
- The market landscape is fragmented, featuring the presence of both established players and new entrants; most medical device label manufacturing companies are located in North America
- Label manufacturers are steadily expanding their capabilities in order to enhance their respective service portfolios and upgrade their capabilities to comply to evolving industry benchmarks
- Digital printing technologies have gradually become on of the much sought-after solutions in this industry; further, there are a variety of upcoming opportunities that are anticipated to sustain growth in this domain
- Acquisitions in this domain are largely driven by efforts to enhance the technology portfolio of label manufacturers; key value drivers include geographical consolidation and capability addition
- Driven by a growing demand for different types of labels, the medical device label manufacturing market is likely to grow at a steady pace over the coming years
- The forecasted future opportunity is anticipated to be distributed across different types of labeling materials used, diverse device classes and application areas
For more information, please visit https://www.rootsanalysis.com/reports/view_document/medical-device-labels-manufacturing-market-2019-2030/272.html
Table of Contents
- PREFACE
- Scope of the Report
- Research Methodology
- Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
- Context and Background
- Overview of Medical Device Packaging
- Overview of Medical Device Labeling
- General Labeling Principles
- In Vitro Diagnostic Devices
- Other Medical Devices
3.4.3 Critical Medical Devices
- Software Used as a Medical Device
- Instructions for End Users
- Good Manufacturing Practices (GMP) Labeling Requirements
- Overview of Labels and Label Manufacturing
- Types of Labels
- Types of Label Printing Techniques
- Types of Label Folding Techniques
- Special Label Features
- Roadblocks to Medical Device Label Manufacturing
- Concluding Remarks
- REGULATORY LANDSCAPE FOR MEDICAL DEVICE LABELING
- Chapter Overview
- Regulatory Guidelines in North America
- The US Scenario
- Canadian Scenario
- Regulatory Guidelines in Europe
- Regulatory Guidelines in Asia Pacific and Rest of the World
- Japanese Scenario
- Chinese Scenario
- Indian Scenario
- MARKET LANDSCAPE
- Chapter Overview
- Medical Device Label Manufacturers
- Analysis by Year of Establishment
- Analysis by Company Size
- Analysis by Location of Headquarters
- Analysis by Regulatory Certifications / Accreditations
- Analysis by Printing Techniques Used
- Analysis by Type of Label Manufactured
- Analysis by Type of Material Used
- Analysis by Additional Label Features
- Analysis by Type of Label Folding
- Analysis by Other Services Offered
- COMPANY PROFILES
- Chapter Overview
- Companies Headquartered in North America
- Avery Dennison
- Company Overview
- Financial Information
- Service Portfolio
- Capabilities
- Future Outlook
- Company Overview
- Service Portfolio
- Future Outlook
- Maverick Label
- Company Overview
- Service Portfolio
- Future Outlook
- Multi-Color
- Company Overview
- Financial Information
- Service Portfolio
- Future Outlook
- Resource Label Group
- Company Overview
- Service Portfolio
- Capabilities
- Future Outlook
- Steven Label
- Company Overview
- Service Portfolio
- Future Outlook
- Topflight
- Company Overview
- Service Portfolio
- Future Outlook
- WS Packaging Group
- Company Overview
- Service Portfolio
- Labeling Equipment
- Future Outlook
- Companies Headquartered in Europe
- Faubel
- Company Overview
- Financial Information
- Service Portfolio
- Future Outlook
- Huhtamaki
- Company Overview
- Financial Information
- Service Portfolio
- Future Outlook
- Matform
- Company Overview
- Service Portfolio
- Future Outlook
- Mondi Group
- Company Overview
- Financial Information
- Service Portfolio
- Future Outlook
- OPM Group
- Company Overview
- Service Portfolio
- Future Outlook
- Schreiner Group
- Company Overview
- Service Portfolio
- Future Outlook
- Companies Headquartered in Asia
- Iwata Label
- Company Overview
- Service Portfolio
- Future Outlook
- Syndicate Label
- Company Overview
- Iwata Label
- Faubel
- Avery Dennison
6.4.2.2 Service Portfolio
- Future Outlook
- MERGERS AND ACQUISITIONS
- Chapter Overview
- Merger and Acquisition Models
- Medical Devices Labeling Companies: Mergers and Acquisitions
- Analysis by Year and Type of Merger and Acquisition
- Analysis by Company Size of Acquired and Acquirer Companies
- Ownership Change Matrix
- Regional Analysis of Merger and Acquisition Activity
- Country-wise Distribution
- Continent-wise Distribution
- Intercontinental and Intracontinental Deals
- Analysis by Key Value Drivers
- Analysis by Key Value Drivers and Year of Acquisition
- Analysis by Markets Served by the Acquired Company
- Analysis by Printing Techniques Used by the Acquired Company
- Analysis by Types of Labels Manufactured by the Acquired Company
- POTENTIAL ACQUISITION TARGETS
- Chapter Overview
- Scope and Methodology
- Potential Strategic Acquisition Targets: Analysis for Resource Label Group
8.3.1 Historical Trend
8.3.2 Top Ten Likely Targets
- Potential Strategic Acquisition Targets: Analysis for ProMach
- Historical Trend
8.4.2 Top Ten Likely Targets
- Potential Strategic Acquisition Targets: Analysis for Huhtamaki
- Historical Trend
8.5.2 Top Ten Likely Targets
- Potential Strategic Acquisition Targets: Analysis for UPM Raflatac
8.6.1 Historical Trend
8.6.2. Top Ten Likely Targets
- Concluding Remarks
- BENCHMARK ANALYSIS
- Chapter Overview
- Methodology
- Region-wise Benchmarking
- North America, Peer Group I
- North America, Peer Group II
- North America, Peer Group III
- Europe, Peer Group IV
- Concluding Remarks
- AC MATRIX
- Chapter Overview
- Scope and Methodology
- Industry Attractiveness Analysis
- Industry Competitiveness Analysis
- GE / McKinsey Matrix
- Concluding Remarks
To purchase a copy, please visit https://www.rootsanalysis.com/reports/view_document/medical-device-labels-manufacturing-market-2019-2030/272.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market is Expected to Grow at a CAGR 20% by 2030
Roots Analysis has done a detailed study on Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market (3rd Edition), 2019-2030: Focus on Circulating Tumor Markers such as CTCs, ctDNA, cfDNA, Exosomes and Other Biomarkers, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
Key Market Insights
- Presently, nearly 300 non-invasive diagnostic tests, designed for the detection of various types of cancers, are either already available in the market or under development across the world
- Several modern diagnostic tests claim to be capable of offering results in relatively short time periods, thereby, facilitating early diagnosis that is particularly beneficial in the treatment of different types of cancers
- Over time, big pharma players have initiated product development programs, having invested significant capital, time and effort, on non-invasive diagnostic solutions for use across different oncological indications
- Investors, having realized the untapped opportunity within this emerging segment of the cancer diagnostics market, have invested over USD 3 billion across 120 instances in the period between 2015 and 2019
- The growing interest in this field is also reflected in the partnership activity; deals inked in the recent past are focused on a diverse range of tumor markers, involving both international and indigenous stakeholders
- The projected future opportunity is anticipated to be driven by increasing patient population and distributed across various disease indications and application areas across key geographies
- These tests are capable of detecting diverse tumor markers that cater to the needs of different stakeholders; in fact, opinions of industry experts confirm the vast potential of liquid biopsies in disease diagnosis / monitoring
For more information, please visit https://www.rootsanalysis.com/reports/view_document/liquid-biopsy-and-nicd-market/279.html
Table of Contents
- PREFACE
- Scope of the Report
- Research Methodology
- Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
- Chapter Overview
- Cancer Statistics and Burden of the Disease
- Importance of Early Cancer Detection
- Cancer Screening and Diagnosis
- Conventional Invasive Cancer Diagnostic Tests
- Biopsy
- Fine Needle Aspiration Biopsy
- Core Needle Biopsy
- Vacuum-Assisted Biopsy
- Image-Guided Biopsy
- Sentinel Node Biopsy
- Surgical Biopsy
- Endoscopic Biopsy
- Bone Marrow Biopsy
- Endoscopy
- Biopsy
- Need for Non-Invasive Approaches
- Liquid Biopsy: Diagnosing Circulating Biomarkers
- Circulating Tumor Cells
- Circulating Tumor DNA
- Exosomes
- Costs and Benefits Associated with Liquid Biopsy and Non-Invasive Tests
- Emerging Trends in Intellectual Property Related to Non-Invasive Cancer Diagnostics
- Challenges Associated with Non-Invasive Cancer Diagnostics
- Future Perspectives
- NON-INVASIVE CANCER SCREENING AND DIAGNOSIS
- Chapter Overview
- Diagnostic Imaging
- Magnetic Resonance Imaging (MRI)
- Mammography
- Bone Scan
- Computerized Tomography (CT) Scan
- Integrated Positron Emission Tomography (PET)-CT Scan
- Ultrasound
- X-ray Radiography (Barium Enema)
- Screening Assays
- Circulating Tumor Marker Test
- Digital Rectal Exam (DRE)
- Fecal Occult Blood Test (FOBT)
- Multigated Acquisition (MUGA) Scan
- Papanicolaou Test and Human Papilloma Virus Test
- Advanced Non-Invasive Approaches
- Cytogenetic / Gene Expression Studies
- Molecular Signature-based Non-Invasive Methods
- Saliva-based Oral Cancer Diagnostics
- Vital Staining
- Optical Biopsy
- Other Diagnostic Techniques
- MARKET LANDSCAPE
- Chapter Overview
- Liquid Biopsy Products: List of Developers
- Analysis by Year of Establishment
- Analysis by Company Size and Geographical Location
- Leading Players
- Analysis by Geography
- Liquid Biopsy Products: List of Available / Under Development Products
- Analysis by Status of Development
- Analysis by Type of Product
- Analysis by Application Area
- Analysis by Target Cancer Indication
- Analysis by Type of Tumor Marker
- Analysis by End User
- Analysis by Turnaround Time
- Liquid Biopsy Products: List of Other Products, Kits and Consumables
- Liquid Biopsy Products: List of Contract Service Providers
- COMPANY PROFILES
- Chapter Overview
- Amoy Diagnostics
- Company Overview
- Financial Information
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- DiaCarta
- Company Overview
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- HaploX
- Company Overview
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- NeoGenomics
- Company Overview
- Financial Information
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- QIAGEN
- Company Overview
- Financial Information
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- Swift Biosciences
- Company Overview
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- Sysmex Inostics
- Company Overview
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- Thermo Fisher Scientific
- Company Overview
- Financial Information
- Liquid Biopsy Product Portfolio
- Recent Developments and Future Outlook
- PARTNERSHIPS AND COLLABORATIONS
- Chapter Overview
- Partnership Models
- List of Partnerships and Collaborations
- Analysis by Year of Partnership
- Analysis by Type of Partnership
- Analysis by Type of Tumor Marker
- Analysis by Target Cancer Indication
- Analysis by Type of Partner
- Most Active Players: Analysis by Number of Partnerships
- Regional Analysis
- Intercontinental and Intracontinental Agreements
- FUNDING AND INVESTMENT ANALYSIS
- Chapter Overview
- Types of Funding
- List of Funding and Investment Instances
- Analysis by Number of Funding Instances
- Analysis by Amount Invested
- Analysis by Type of Funding
- Analysis by Target Cancer Indication
- Analysis by Type of Tumor Marker
- Most Active Players: Analysis by Amount Invested
- Most Active Investors: Analysis by Number of Funding Instances
- Regional Analysis by Amount Invested
- Concluding Remarks
- LIQUID BIOPSY: INITIATIVES OF BIG PHARMA PLAYERS
- Chapter Overview
- Top Pharmaceutical Companies
- Analysis by Status of Development
- Analysis by Type of Tumor Marker
- Analysis by Application Area
- Analysis by Target Cancer Indication
- KEY ACQUISITION TARGETS
- Chapter Overview
- Scope and Methodology
- Scoring Criteria and Key Assumptions
- Potential Strategic Acquisition Targets in North America
- Potential Strategic Acquisition Targets in Europe
- Potential Strategic Acquisition Targets in Asia-Pacific / Rest of the World
- Concluding Remarks
- OTHER NON-INVASIVE CANCER DIAGNOSTICS
- Chapter Overview
- Non-Blood-based Biomarker Detection Tests
- FOBT and Fecal Immunochemical Tests (FIT)
- Pigmented Lesion Assays
- Stool DNA (sDNA)-based Tests
- Volatile Organic Compound (VOC) Detection Tests
- Other Non-Invasive Cancer Diagnostics: Market Landscape
- MARKET SIZING AND OPPORTUNITY ANALYSIS
- Chapter Overview
- Key Assumptions and Forecast Methodology
- Global Non-Invasive Cancer Diagnostics Market, 2019-2030
- Global Liquid Biopsy Market, 2019-2030
- Global Liquid Biopsy Market: Distribution by Application Area, 2019-2030
- Global Liquid Biopsy Market for Early Diagnosis, 2019-2030
- Global Liquid Biopsy Market for Patient Monitoring, 2019-2030
- Global Liquid Biopsy Market for Recurrence Monitoring, 2019-2030
- Global Liquid Biopsy Market: Distribution by Target Cancer Indication, 2019-2030
- Global Liquid Biopsy Market for Breast Cancer, 2019-2030
- Global Liquid Biopsy Market for Lung Cancer, 2019-2030
- Global Liquid Biopsy Market for Colorectal Cancer, 2019-2030
- Global Liquid Biopsy Market for Prostate Cancer, 2019-2030
- Global Liquid Biopsy Market for Bladder Cancer, 2019-2030
- Global Liquid Biopsy Market for Melanoma, 2019-2030
- Global Liquid Biopsy Market for Gastric Cancer, 2019-2030
- Global Liquid Biopsy Market for Pancreatic Cancer, 2019-2030
- Global Liquid Biopsy Market for Ovarian Cancer, 2019-2030
- Global Liquid Biopsy Market: Distribution by Type of Tumor Marker, 2019-2030
- Global Liquid Biopsy Market for ctDNA, 2019-2030
- Global Liquid Biopsy Market for cfDNA, 2019-2030
- Global Liquid Biopsy Market for CTCs, 2019-2030
- Global Liquid Biopsy Market for Exosomes, 2019-2030
- Global Liquid Biopsy Market for Other Tumor Markers, 2019-2030
- Global Liquid Biopsy Market: Distribution by Type of Analyte, 2019-2030
- Global Liquid Biopsy Market for Blood, 2019-2030
- Global Liquid Biopsy Market for Other Body Fluids, 2019-2030 (USD Billion)
- Global Liquid Biopsy Market: Distribution by End User, 2019-2030
- Global Liquid Biopsy Market for Hospitals, 2019-2030
- Global Liquid Biopsy Market for Research Institutes, 2019-2030
- Global Liquid Biopsy Market for Other End Users, 2019-2030
- Global Liquid Biopsy Market: Distribution by Geography, 2019-2030
- Liquid Biopsy Market in the US, 2019-2030
- Liquid Biopsy Market in the UK, 2019-2030
- Liquid Biopsy Market in Germany, 2019-2030
- Liquid Biopsy Market in France, 2019-2030
- Liquid Biopsy Market in Italy, 2019-2030
- Liquid Biopsy Market in Spain, 2019-2030
- Liquid Biopsy Market in Japan, 2019-2030
- Liquid Biopsy Market in China, 2019-2030
- Liquid Biopsy Market in India, 2019-2030
- Liquid Biopsy Market in Australia, 2019-2030
- Other Non-Invasive Cancer Diagnostics Market Forecast, 2019-2030
- Global Liquid Biopsy Market: Distribution by Application Area, 2019-2030
- SURVEY INSIGHTS
- Chapter Overview
- Company Specifics of Respondents
- Designation of Respondents
- Type of Product Portfolio
- Types of Products / Services Offered
- Application Area
- Status of Development of the Products
- Likely Market Size
- CONCLUSION
- Timely Disease Detection and Subsequent Monitoring are Critical Elements of Patient Care
- in the Field of Oncology
- Introduction of Sophisticated Molecular Diagnostics has Facilitated Better Cancer
- Management
- Liquid Biopsy has Emerged as a Reliable Alternative to the Invasive Methods of Diagnosis
- The Versatile and Patient Friendly Nature of these Diagnostic Tools Cater to a Wide Range
- of Applications
- The Interest is Gradually Rising with Participation of Several Start-ups Across Different
- Geographies
- In Addition to Liquid Biopsy, Development of Other Non-Invasive Tests will Further
- Strengthen the Ongoing Innovation
- Rising Venture Capital Support is Indicative of a Lucrative Future Potential
- Primarily Led by Liquid Biopsy, the Non-Invasive Cancer Diagnostics Market has Emerged
- as a Multi-Billion Dollar Market
- EXECUTIVE INSIGHTS
- Chapter Overview
- Interview Transcript: Shibichakravarthy Kannan, Founder & Chief Executive Officer,
- Theranosis Life Sciences
- Interview Transcript: Anton Iliuk, President and Chief Technology Officer, Tymora Analytical
- Operations
- Interview Transcript: Peter French, Strategic Technology Advisor, Sienna Cancer
- Diagnostics
- Interview Transcript: Joachim Fluhrer, Founder and Medical Director, Genostics
- Interview Transcript: Brad Walsh, Chief Executive Officer, Minomic International
- Interview Transcript: Catalina Vasquez, Chief Operating Officer, Nanostics
- Interview Transcript: Burkhard Jansen, Chief Medical Officer, DermTech
- Interview Transcript: Frank Szczepanski, President and CEO, IVDiagnostics
- Interview Transcript: Riccardo Razzini, Sales and Marketing Manager, LCM Genect
- Interview Transcript: Nathalie Bernard, Marketing Director, OncoDNA
- Interview Transcript: Abizar Lakdawalla, Founder, Proxeom
- Interview Transcript: Mark Li, CEO, Resolution Bioscience
- Interview Transcript: Christer Ericsson, Chief Scientific Officer, iCellate Medical
- Interview Transcript: Philippe Nore, CEO and Co-founder, MiNDERA
- Interview Transcript: Jake Micallef, Chief Scientific Officer, VolitionRx
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
- Timely Disease Detection and Subsequent Monitoring are Critical Elements of Patient Care
- Global Liquid Biopsy Market, 2019-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Cell and Advanced Therapies Supply Chain Management Market Report – Industry Size, Share, Trends, Growth and Forecast Till 2030
The cell and advanced therapies supply chain is complex, with several legacy challenges, such as those related to patient scheduling, resource planning, inventory management, and deliverable tracking. A number of innovative, software-enabled systems are available / under development to mitigate the aforementioned concerns and simplify the management of biopharmaceutical supply chains.
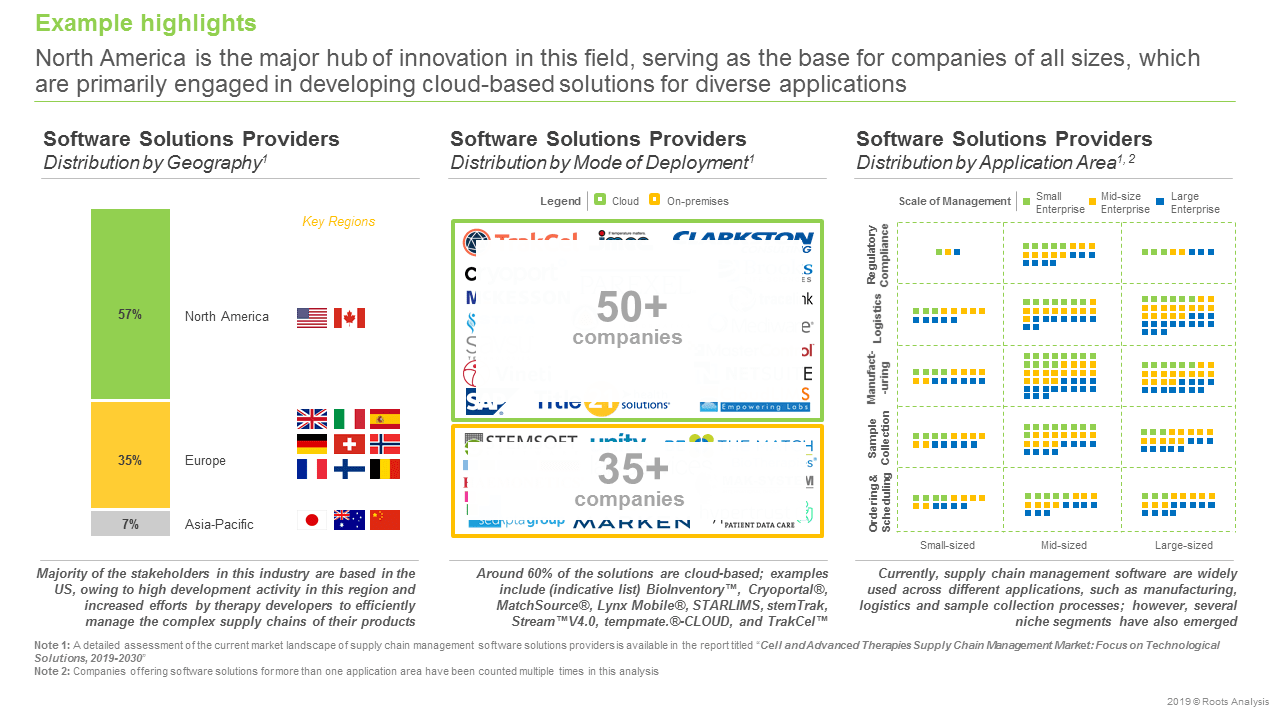
The USD 1.5 billion (by 2030) financial opportunity within the cell and advanced therapy supply chain management software solutions market has been analysed across the following segments:
Application area
- Sample collection and processing
- Manufacturing
- Logistics
- Patient identification and treatment follow-up
Type of software solution
- Cell orchestration platform
- Enterprise manufacturing system
- Inventory management system
- Laboratory information management system
- Logistics management system
- Patient management system
- Quality management system
Mode of Deployment
- Cloud-based solution
- On-premises solution
Scale of Operation
- Clinical
- Commercial
End user
- Biobank
- Cell therapy lab / commercial organization
- Hospital / medical center
- Research institute
Key geographical regions
- North America
- Europe
- Asia Pacific
- Rest of the world
The Cell and Advanced Therapies Supply Chain Management Market: Focus on Technological Solutions (Cell Orchestration Platforms, Enterprise Manufacturing Systems, Inventory Management Systems, Laboratory Information Management Systems, Logistics Management Systems, Patient Management Systems, Quality Management Systems, Tracking & Tracing Systems, and Other Software), 2019-2030 report features the following companies, which we identified to be key players in this domain:
key players
- Be The Match BioTherapies®
- Brooks Life Sciences
- Clarkston Consulting
- Cryoport
- Haemonetics
- Hypertrust Patient Data Care
- Lykan Bioscience
- MAK-SYSTEM
- MasterControl
- SAP
- SAVSU Technologies
- sedApta Group
- Stafa Cellular Therapy
- Title21 Health Solutions
- TraceLink
- TrakCel
- Vineti
Table of Contents
- Preface
- Executive Summary
- Introduction
- Current Market Landscape
- Company Competitiveness Analysis
- Cell and Advanced Therapies Supply Chain Management: Company Profiles
- Supply Chain Orchestration Platform: Emerging Trends and Key Players
- Funding and Investment Analysis
- Partnerships and Collaborations
- Supply Chain Utilization Use Cases
- Stakeholder Needs Analysis
- Cost Savings Analysis
- Market Forecast
- Executive Insights
- Concluding Remarks
- Appendix 1: List of Additional Supply Chain Management Software Solutions
- Appendix 2: Tabulated Data
- Appendix 3: List of Companies and Organizations
To purchase a copy, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapies-supply-chain/260.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Global Medical Device Label Contract Manufacturing Market Report Forecast by Development, Trends, and Forecast (2019 – 2030)
Outsourcing the manufacturing of labels is a popular trend among medical device developers, enabling such companies to leverage the advanced capabilities of CMOs, while focusing on the core aspects of product development
Roots Analysis has announced the addition of “Medical Device Labels Technology Market, 2019-2030” report to its list of offerings.
The report features an extensive study of the current market landscape of companies offering manufacturing services for medical device labels. The study also features detailed analysis and an elaborate discussion on the future potential of this evolving market. Amongst other elements, the report includes:
- A detailed assessment of the overall landscape of companies offering manufacturing services for medical device labels.
- A detailed analysis of the various mergers and acquisitions that have taken place in this domain, highlighting the key value drivers of such deals inked between 2014 and 2019 (till June).
- A detailed acquisition target analysis, which takes into consideration the historical trend of activity of top acquirers, providing a means for industry stakeholders to identify potential acquisition targets.
- An industry-wide benchmark analysis, highlighting the key focus areas of small, mid-sized and large companies.
- A detailed business portfolio analysis based on the 9-box attractiveness and competitiveness (AC) matrix framework, highlighting the current market attractiveness and competitive strength of different printing technologies used by label manufacturers.
- An elaborate discussion on the various guidelines established by major regulatory bodies, governing medical device labelling-related practices and product approval, across different countries.
- Elaborate profiles of key players that claim to have a diverse range of capabilities for the manufacturing of different types of labels for medical devices.
- A discussion on important industry-specific trends, key market drivers and challenges, under a SWOT framework.
- A review of the various upcoming opportunities and anticipated future trends related to medical device label manufacturing that are expected to influence the evolution of this industry over the coming years.
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Get Detailed Analysis of this Research Reports at: Medical Device Labels Technology Market, 2019-2030
Type of labels
- Glue applied labels
- Pressure sensitive labels
- In mould labels
- Shrink sleeve labels
- Other labels
Type of material
- Plastic labels
- Paper labels
- Other material labels
Application type
- Monitoring and diagnostic equipment labels
- Disposable consumables labels
- Therapeutic equipment labels
- Device class
- Class I medical devices
- Class II medical devices
- Class III medical devices
Key geographical regions
- North America
- Europe
- Asia Pacific
Key companies covered in the report
- Avery Dennison
- Faubel
- Huhtamaki
- Iwata Label
- Labeltape
- Matform
- Maverick Label
- Mondi Group
- Multi-Color
- OPM Group
- Resource Label Group
- Schreiner Group
- Steven Label
- Topflight
- WS Packaging
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Targeted Protein Degradation Market is Expected to Grow at a CAGR 30% by 2030
Roots Analysis has done a detailed study on Targeted Protein Degradation Market: Focus on Therapeutics and Technology Platforms (based on Degronimids, ENDTACs, Epichaperome Inhibitors, Hydrophobic Tags, IMiDs, LYTACs, Molecular Glues, PHOTACs, PROTACs, Protein Homeostatic Modulators, SARDs, SERDs, SNIPERs, and Specific BET and DUB Inhibitors), 2020-2030, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
Key Market Insights
- More than 85 small molecule protein degraders are currently being evaluated for the treatment of various disease indications; in addition, there are 25+ technology platforms available for use in therapy development efforts
- The pipeline features a variety of candidate drugs that target a wide range of disease-causing / associated proteins; majority of the existing drug candidates are designed for administration via non-invasive routes
- Although start-ups and mid-sized firms are spearheading the innovation, several big pharmaceutical companies are also engaged in this domain
- Close to 5,500 patients were estimated to have been enrolled in clinical trials worldwide, evaluating a number of relevant pre-marketing end points across various phases of development
- A number of prominent scientists from renowned universities have emerged as key opinion leaders, owing to their active involvement in clinical development efforts
- Published scientific literature indicates that both industry and academic players have made equal contributions to the innovation in this field; the major focus of such studies is presently on PROTACs
- Foreseeing a lucrative future, several private and public investors have invested over USD 3.5 billion across close to 100 instances of funding since 2014
- The increasing interest in this field is also reflected in recent partnership activity; most of these deals are focused on novel technology platforms, involving the active participation of both international and indigenous companies
- Short term opportunity in this market is likely to be driven by licensing activity, depending on the capability of novel technologies to meet protein degrader design and development needs
- As multiple mid-late stage drug candidates are approved for marketing, the long term opportunity is likely to be distributed across different types of protein degraders, target therapeutic areas and various global regions
For more information, please visit:
https://www.rootsanalysis.com/reports/view_document/protein-degradation-market/289.html
Table of Contents
- PREFACE
- Scope of the Report
- Research Methodology
- Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
- Context and Background
- Concept of Protein Homeostasis
- Discovery of the Ubiquitin Proteasome System
- Ubiquitin: Structure and Function
- Fundamentals of the UPS
- Components of the UPS
- Key Steps Involved in the UPS
- Ubiquitination: The First Step
- Proteasomal Degradation: The Second Step
- Therapeutic Applications of the UPS
- Advantages and Challenges Associated with Ubiquitin Enzyme Inhibitors
- Targeted Protein Degradation: Enhancing Ubiquitination to Degrade Undruggable Targets
- Brief History of Targeted Protein Degradation
- Types of Protein Degraders
- Selective Hormone Receptor Degraders (SHRDs)
- Immumodulatory Imide Drugs (IMiDs)
- PROTACs
- Other Chimeras (ENDTACs, LYTACs and PHOTACs)
- Endosome Targeting Chimeras (ENDTACs)
- Lysozyme targeting chimeras (LYTACs)
- Specific and Nongenetic Inhibitor-of-Apoptosis Proteins (IAP)-dependent Protein Erasers (SNIPERS)
- Hydrophobic Tag
- Molecular Glues
- DUB Inhibitors
- Growth Drivers and Roadblocks
- CURRENT MARKET LANDSCAPE
- Chapter Overview
- Targeted Protein Degradation-based Therapeutics and Technologies: Development Pipeline
- Analysis by Type of Protein Degrader
- Analysis by Phase of Development
- Analysis by Therapeutic Area
- Analysis by Target Indication
- Analysis by Type of Target Enzyme
- Analysis by Type of Target Protein
- Analysis by Type of Therapy
- Analysis by Route of Administration
- Targeted Protein Degradation-based Therapeutics and Technologies: List of Research Tools / Key Technology Platforms
- Targeted Protein Degradation-based Therapeutics and Technologies: Developer Landscape
- Analysis by Year of Establishment
- Analysis by Location of Headquarters
- Analysis by Size of Company
- Analysis by Type of Protein Degrader
- COMPANY PROFILES
- Chapter Overview
- Developers with Clinical Candidates
- Radius Health
- Company Overview
- Targeted Protein Degradation-based Drug Portfolio
- Product Description: Elacestrant
- Recent Developments and Future Outlook
- Celgene
- Company Overview
- Financial Information
- Targeted Protein Degradation-based Drug Portfolio
- Avadomide (CC-122)
- Iberdomide (CC-220)
- Recent Developments and Future Outlook
- Sanofi Genzyme
- Company Overview
- Financial Information
- Targeted Protein Degradation-based Drug Portfolio
- Product Description: SAR439859
- Recent Developments and Future Outlook
- Developers with Preclinical / Early-stage Clinical Candidates
- Arvinas
- Captor Therapeutics
- Genentech
- Kymera Therapeutics
- Mission Therapeutics
- Progenra
- Zenopharm
- Radius Health
- CLINICAL TRIAL ANALYSIS
- Chapter Overview
- Scope and Methodology
- Targeted Protein Degradation-based Therapeutics and Technologies: List of Clinical Trials
- Analysis by Trial Registration Year
- Geographical Analysis by Number of Clinical Trials
- Geographical Analysis by Enrolled Patient Population
- Analysis by Type of Protein Degrader
- Analysis by Phase of Development
- Analysis by Study Design
- Analysis by Type of Sponsor / Collaborator
- Most Active Players: Analysis by Number of Registered Trials
- Analysis by Trial Focus
- Analysis by Therapeutic Area
- Analysis by Clinical Endpoints
- KOL ANALYSIS
- Chapter Overview
- Scope and Methodology
- Targeted Protein Degradation-based Therapeutics and Technologies: List of Principal Investigators Involved in Clinical Trials
- Analysis by Type of Organization
- Analysis by Designation
- Geographical Distribution
- Analysis by Therapeutic Focus
- Analysis by Phase of Development and Type of Degrader
- Prominent Key Opinion Leaders
- KOL Benchmarking: Roots Analysis versus Third Party Scoring (ResearchGate Score)
- Most Active Key Opinion Leaders
- KOL Profile (Hagop Youssoufian)
- KOL Profile (Patricia LoRusso)
- KOL Profile (Johann De Bono)
- KOL Profile (John N Nemunaitis)
- KOL Profile (Robert Morgan)
- KOL Profile (Edward O’Mara)
- PUBLICATION ANALYSIS
- Chapter Overview
- Scope and Methodology
- Targeted Protein Degradation-Based Therapeutics and Technologies: Recent Publications
- Analysis by Year of Publication
- Analysis by Study Objective
- Emerging Focus Areas
- Analysis by Type of Protein Degrader
- Analysis by Target Protein
- Analysis by Target Enzyme
- Analysis by Target Indication
- Analysis by Type of Publisher
- Leading Players: Analysis by Number of Publications
- Leading Players: Geographical Analysis by Number of Publications
- Key Journals: Analysis by Number of Publications
- FUNDING AND INVESTMENT ANALYSIS
- Chapter Overview
- Types of Funding
- Targeted Protein Degradation: Funding and Investment Analysis
- Analysis by Number of Funding Instances
- Analysis by Amount Invested
- Analysis by Type of Funding
- Analysis by Number of Funding Instances and Amount Invested across Different Protein Degraders
- Analysis by Number of Funding Instances and Amount Invested across Different Therapeutic Areas
- Analysis by Amount Invested across Different Protein Degradation Technology Platforms
- Most Active Players: Analysis by Number of Funding Instances
- Most Active Investors: Analysis by Number of Funding Instances
- Geographical Analysis by Amount Invested
- Concluding Remarks
- PARTNERSHIPS AND COLLABORATIONS
- Chapter Overview
- Partnership Models
- Targeted Protein Degradation-based Therapeutics and Technologies: Recent Collaborations and Partnerships
- Analysis by Year of Partnership
- Analysis by Type of Partnership
- Analysis by Type of Protein Degrader
- Analysis by Therapeutic Area
- Analysis by Different Protein Degradation Technology
- Most Active Players: Analysis by Number of Partnerships
- Geographical Analysis
- Most Active Players: Regional Analysis by Number of Partnerships
- Intercontinental and Intracontinental Agreements
- MARKET SIZING AND OPPORTUNITY ANALYSIS
- Chapter Overview
- Key Assumptions and Forecast Methodology
- Overall Targeted Protein Degradation-based Therapeutics and Technologies Market, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market by Upfront Payments, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market by Milestone Payments, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market: Distribution by Type of Protein Degrader
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Degronimids, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for PROTACs, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for SARDs / SERDs, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Specific BET and DUB Inhibitors, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Other Protein Degraders, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market: Distribution by Therapeutic Area
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Neurodegenerative Disorders, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Oncological Disorders, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Other Therapeutic Areas, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market: Distribution by Route of Administration
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Oral Route, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Intravenous Route, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market for Other Routes, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market: Distribution by Geography
- Targeted Protein Degradation-based Therapeutics and Technologies Market in North America, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market in Europe, 2020-2030
- Targeted Protein Degradation-based Therapeutics and Technologies Market in Asia-Pacific, 2020-2030
- EXECUTIVE INSIGHTS
- CONCLUDING REMARKS
- Chapter Overview
- Key Takeaways
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Subcutaneous Biologics, Technologies and Drug Delivery Systems Market 2019 | Global Forecast 2030
Subcutaneous drug delivery, given the use of tailored formulation development solutions, has brought about a paradigm shift in at-home healthcare, enabling users to administer (life-saving) medications without having to rely on medical professionals
Roots Analysis is pleased to announce the publication of its recent study, titled “Subcutaneous Biologics, Technologies and Drug Delivery Systems, 2020-2030” report to its list of offerings.
The report provides a detailed study on the current market landscape and future potential of biologics designed for subcutaneous administration. In addition, the study provides an in-depth analysis of the formulation technologies and drug delivery systems (focusing on large volume wearable injectors, autoinjectors, pen injectors, needle-free injectors, drug reconstitution systems, prefilled syringes and implants) that enable subcutaneous delivery of the biologic drugs. Amongst other elements, the report features the following:
- A detailed assessment of the current market landscape of commercially available and clinical-stage biologics that are designed for delivery via the subcutaneous route
- A case study on leading subcutaneous biologics (in terms of revenues generated), featuring details on mechanism of action, development history, annual sales, technology platform (if available), and a comparison of their intravenous and subcutaneous formulations (if applicable).
- An assessment of the various subcutaneous formulation technologies along with information on developers, type of pharmacological molecule, route of administration, mechanisms of action and primary advantage(s).
- An insightful three-dimensional comparison of the subcutaneous formulation technology developers, based on pipeline strength, supplier power of the developer and primary advantages offered by their respective technologies.
- Elaborate profiles of key technology developers, featuring a brief overview of the company, its technology portfolio, product portfolio, financial information (if available), recent developments and an informed future outlook.
- An analysis of collaborations and partnership agreements inked by the subcutaneous formulation technology developers since 2011
- An in-depth review of the most advanced and popular subcutaneous drug delivery systems, including large volume wearable injectors, autoinjectors, pen injectors, needle-free injectors, drug reconstitution systems, prefilled syringes and implants
- A comprehensive product competitiveness analysis of subcutaneous large volume wearable injectors, subcutaneous autoinjectors, subcutaneous needle-free injectors and pre-filled syringes
- A discussion on affiliated trends, key drivers and challenges, which are likely to impact the industry's evolution, under a comprehensive SWOT framework
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below):
Market Segments
Phase of development
- Approved
- Pre-registration & Phase III
- Phase II & Phase II/III
Type of molecule
- Cell and gene therapies
- Monoclonal antibodies
- Proteins
- Peptides (recombinant)
- Vaccines
- Others
Target therapeutic area
- Autoimmune disorders
- Blood disorders
- Bone disorders
- Genetic disorders
- Metabolic disorders
- Neurological disorders
- Oncological disorders
- Respiratory disorders
- Others
Type of drug delivery system
- Large volume wearable injectors
- Autoinjectors
- Prefilled syringes
- Needle-free injectors
- Drug reconstitution systems
Revenues from licensing deals
- Upfront payments
- Milestone payments
Key geographical regions
- North America
- Europe
- Asia Pacific
- Rest of the World
Transcripts of interviews held with the following senior level representatives of stakeholder companies
- Deborah Bitterfield (Founder and Chief Executive Officer, Lindy Biosciences)
- Matthew Young (Founder and Chief Technology Officer, Oval Medical Technologies)
- Steve Prestrelski (Founder and Chief Scientific Officer, Xeris Pharmaceuticals), Hong Qi (Vice President, Product Development, Xeris Pharmaceuticals) and Scott Coleman (Senior Scientist, Formulation, Xeris Pharmaceuticals)
- David Daily (Co-Founder and Chief Executive Officer, DALI Medical Devices)
- Michael Reilly (Co-Founder and Chief Executive Officer and Co-Founder, Excelse Bio)
- Poonam R Velagaleti (Co-Founder, i-novion)
- Michael Hooven (Chief Executive Officer, Enable Injections)
- Frederic Ors (Chief Executive Officer, Immunovaccine Technologies)
- Patrick Anquetil (Chief Executive Officer, Portal Instruments)
- Menachem Zucker (Vice President and Chief Scientist, Elcam Medical)
- Tiffany H. Burke (Director, Global Communications, West Pharmaceutical Services) and Graham Reynolds (Vice President and General Manager, Global Biologics, West Pharmaceutical Services)
- David Heuzé (Communication Leader, MedinCell)
Key companies covered in the report
- Adocia
- Ajinomoto Bio-Pharma Services
- Arecor
- Alteogen
- Ascendis Pharma
- Avadel Pharmaceuticals
- Camurus
- Creative BioMart
- Creative Biolabs
- DURECT
- Eagle Pharmaceuticals
- Halozyme Therapeutics
- MedinCell
- Xeris Pharmaceuticals
- Serina Therapeutics
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/subcutaneous-biologics-delivery/314.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com

Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market Research Report 2019-2030
Liquid biopsies have emerged as a versatile diagnostic solution, especially for cases when conventional (invasive) diagnostic tests are inconclusive or when the clinical condition is related to an organ that is not amenable to biopsy access
Roots Analysis is pleased to announce the publication of its recent study, titled, “Liquid Biopsy and Other Non-Invasive Cancer Diagnostics Market, 2019-2030: Focus on Circulating Tumor Markers such as CTCs, ctDNA, cfDNA, Exosomes and Other Biomarkers.”
The report features an extensive study of the current market landscape, offering an informed opinion on the likely adoption of this approach over the next decade. It features an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain. In addition to other elements, the study includes:
- A detailed review of the overall landscape of the non-invasive cancer diagnostics market.
- An analysis of the various partnerships pertaining to non-invasive cancer diagnostics, which have been established between 2016 and 2019.
- An analysis of the investments made in companies engaged in the development of non-invasive cancer diagnostics.
- An analysis of the initiatives of big pharma players.
- A detailed acquisition target analysis.
- Elaborate profiles of the key players engaged in this domain.
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Market Segments
Type of Tumor Marker
- ctDNA
- cfDNA
- CTCs
- Exosomes
- Others
Application
- Diagnosis / Early Diagnosis
- Patient Monitoring
- Recurrence Monitoring
Target Cancer Indication
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Bladder Cancer
- Melanoma
- Gastric Cancer
- Pancreatic Cancer
- Ovarian Cancer
- Others
End Users
- Hospitals
- Research Institutes
- Others
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Key companies covered in the report:
- Amoy Diagnostics
- DiaCarta
- HaploX Biotechnology
- NeoGenomics
- QIAGEN
- Swift Biosciences
- Sysmex Inostics
- Thermo Fisher Scientific
For more information, please click on the following link:
https://www.rootsanalysis.com/reports/view_document/liquid-biopsy-and-nicd-market/279.html
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com

Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
Cell and Advanced Therapies Supply Chain Management Market: Global Industry Analysis and Opportunity Assessment, 2019 – 2030
Roots Analysis has done a detailed study on Cell and Advanced Therapies Supply Chain Management Market: Focus on Technological Solutions (Cell Orchestration Platforms, Enterprise Manufacturing Systems, Inventory Management Systems, Laboratory Information Management Systems, Logistics Management Systems, Patient Management Systems, Quality Management Systems, Tracking & Tracing Systems, and Other Software), 2019-2030, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
To order this 510+ page report, which features 185+ figures and 400+ tables, please visit this link
Key Market Insights
- Presently, over 160 innovative, software-enabled systems are being used to efficiently manage and streamline various aspects of the complex supply chain of cell and advanced therapies
- Company involved in this domain are putting in significant efforts to develop advanced software solutions and also differentiate their offerings, from those of other industry players, in order to maintain a competitive edge
- North America is the major hub of innovation in this field, serving as the base for companies of all sizes, which are primarily engaged in developing cloud-based solutions for diverse applications
- The cell and advanced therapies market is characterized by an elaborate value chain, involving a multitude of processes and several stakeholders, each having a discrete set of priorities and requirements
- Increase in partnership activity reflects the growing interest of stakeholders in this industry; over 50% of reported deals were established to deploy software solutions for enhancing visibility and supply chain performance
- An analysis of recent activity on Twitter reveals the increasing interest and ongoing efforts of industry stakeholders in providing needle-to-needle traceability and supply chain orchestration solutions
- Given their cost saving potential across different processes and operations, we expect the cell and advanced therapy supply chain solutions market to grow at an annualized rate of around 25% over the next decade
- In the mid-long term, the projected opportunity is anticipated to be well distributed across various global regions, different end uses / applications and mode of deployment of various proprietary software solutions
For more information, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapies-supply-chain/260.html
Table of Contents
- PREFACE
- EXECUTIVE SUMMARY
- INTRODUCTION
- CURRENT MARKET LANDSCAPE
- COMPANY COMPETITIVENESS ANALYSIS
- CELL AND ADVANCED THERAPIES SUPPLY CHAIN MANAGEMENT: COMPANY PROFILES
- SUPPLY CHAIN ORCHESTRATION PLATFORM: EMERGING TRENDS AND KEY PLAYERS
- FUNDING AND INVESTMENT ANALYSIS
- PARTNERSHIPS AND COLLABORATIONS
- SUPPLY CHAIN UTILIZATION USE CASES
- STAKEHOLDER NEEDS ANALYSIS
- COST SAVINGS ANALYSIS
- MARKET FORECAST
- EXECUTIVE INSIGHTS
- CONCLUDING REMARKS
- APPENDIX 1
- APPENDIX 1
- APPENDIX 3
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
